- Overview of Checkpoint Inhibitors:
- Checkpoint inhibitors are humanized monoclonal antibodies that activate T-cells to target tumor cell antigens, first FDA-approved in 2011.
- Common agents: pembrolizumab, ipilimumab, nivolumab (recognizable by “-mab” suffix).
- Treat a wide range of cancers, including cutaneous melanoma, lung cancer, and gastrointestinal/genitourinary cancers, with objective response rates up to 60–70%.
- Revolutionized oncology by leveraging the immune system, particularly for cancers with previously limited treatment options.
- Ocular Side Effects Prevalence:
- Ocular adverse events occur in 1–3% of patients in clinical trials, but a retrospective MD Anderson study reported up to 10% prevalence when pooling data across all checkpoint inhibitors over 10 years.
- Ophthalmologists must be vigilant due to the increasing use of these agents and their cumulative ocular effects.
- Mechanisms of Ocular Toxicity:
- Checkpoint inhibitors disrupt immune privilege, particularly by targeting PD-L1 on retinal pigment epithelium (RPE), which normally suppresses aberrant T-cells in the eye.
- In melanoma patients, lysis of melanoma cells releases melanin-associated proteins, triggering a Vogt-Koyanagi-Harada (VKH)-like uveitis due to cross-reactivity with melanosome-rich tissues (e.g., uvea).
- Ocular side effects mimic autoimmune diseases, affecting any part of the eye (orbit to retina).
- Common Ocular Side Effects:
- Anterior Segment:
- Dry eye disease: Common, under-reported, and exacerbated in cancer patients; may mimic Sjogren’s-like ocular surface disease.
- Anterior uveitis/iritis: Often linked to PD-L1 inhibition, presenting with cells, flare, or conjunctival inflammation.
- Keratitis: Beyond dry eye, may involve corneal inflammation.
- Episcleritis/scleritis: Inflammatory changes mimicking autoimmune conditions.
- Posterior Segment:
- Posterior uveitis/panuveitis: May present with vitreous cell, resembling VKH (e.g., serous retinal detachments).
- VKH-like syndrome: Characterized by vitiligo, uveitis, and serous detachments, especially in melanoma patients.
- Other:
- Orbital inflammatory disease.
- Vitreous infiltration mimicking inflammation but potentially due to cancer cells (non-responsive to checkpoint inhibitors in the immune-privileged eye).
- Anterior Segment:
- Diagnostic Challenges:
- No characteristic presentation; ocular side effects mimic autoimmune or infectious etiologies, requiring a broad differential.
- Rule out common causes first: HLA-B27-associated uveitis, syphilis, sarcoidosis, Lyme disease, and viral infections (e.g., HSV, due to immunosuppression).
- Clues to checkpoint inhibitor-related etiology:
- Temporal correlation with dosing (symptoms 2–7 days post-dose, resolving before the next dose).
- Concurrent systemic inflammatory events (e.g., GI inflammation).
- Vitreous infiltration may represent cancer cells, not inflammation, necessitating biopsy in unclear cases (e.g., diagnostic vitrectomy or anterior chamber tap).
- Baseline ophthalmic exams are ideal but not always feasible; community ophthalmologists should be proactive in evaluating referred patients.
- Management Strategies:
- Goal: Minimize systemic immunosuppression to preserve the anti-cancer effect of checkpoint inhibitors.
- Anterior Segment:
- Topical corticosteroids (e.g., prednisolone acetate) for dry eye, keratitis, episcleritis, or mild uveitis; often sufficient without dose adjustment.
- Cycloplegics for anterior uveitis to prevent synechiae.
- Posterior Segment:
- Intravitreal corticosteroids (e.g., Ozurdex, Yutiq) preferred over periocular injections for posterior uveitis or macular edema, based on clinical trial data (e.g., POINT trial).
- Short bursts of low-dose oral prednisone (≤10 mg/day) if local therapy is insufficient, tapered quickly to avoid dampening anti-cancer effects.
- Systemic Immunomodulatory Therapy (IMT):
- Rarely used (e.g., methotrexate for inflammatory arthritis); avoided unless multi-organ involvement requires it, due to immune suppression risks.
- Coordination with Oncologists:
- Essential to balance ocular and oncologic outcomes; involves discussing dose delays, reductions, or pre-treatment with steroids.
- Stopping checkpoint inhibitors is rare for ocular side effects alone unless severe, as patients prioritize cancer control.
- When to Stop Checkpoint Inhibitors:
- Discontinuation is uncommon unless multi-organ toxicity or intolerable systemic side effects occur.
- If ocular inflammation is severe but isolated, local therapy and dose adjustments (e.g., reducing dose or extending intervals) are preferred.
- Patients may refuse discontinuation if the drug is life-sustaining, necessitating aggressive local management.
- Treatment Duration and Switching Agents:
- No defined stopping point; typical course is ~1 year, but patients with partial response may continue indefinitely.
- Switching checkpoint inhibitors is rare after severe toxicity; protocols often exclude patients with prior toxicity from new trials.
- Dual-agent therapy (e.g., CTLA-4 + PD-1 inhibitors) may be de-escalated to a single agent to reduce toxicity while maintaining efficacy.
- Newer agents targeting different immune pathways may offer flexibility in the future.
- Practical Recommendations:
- Baseline ophthalmic exams are valuable for establishing a reference, especially in academic centers or clinical trials.
- Community ophthalmologists should promptly evaluate patients referred for ocular symptoms, advising oncologists to continue therapy until assessment.
- Work closely with oncologists to tailor treatment, avoiding unnecessary discontinuation of life-saving therapy.
- Resource: National Comprehensive Cancer Network (NCCN) algorithm for managing immune-related adverse events, updated annually (link in podcast description).
- Board-Relevant Takeaways:
- Recognize checkpoint inhibitors by their “-mab” suffix and associate them with a 1–10% risk of ocular side effects.
- VKH-like uveitis is a hallmark in melanoma patients due to melanin-associated protein release.
- Differential diagnosis must include infectious and autoimmune etiologies, with syphilis, HSV, and cancer infiltration as key considerations.
- Local therapy (topical/intravitreal steroids) is first-line to minimize systemic immunosuppression; systemic steroids are limited to ≤10 mg/day.
- Never assume vitreous cell is inflammatory; biopsy may be needed to rule out cancer infiltration.
Citation of the Podcast
Young, B., Berkenstock, M., Dalvin, L., & Kim, S. (Host). (2025). Checkpoint inhibitors and their ocular side effects. Experts InSight [Podcast]. American Academy of Ophthalmology. Available at: aao.org/podcasts. Additional resource: National Comprehensive Cancer Network (NCCN) Immune-related Adverse Events Panel algorithm (link provided in podcast description).
- Patient Presentation: A man in his 50s with metastatic clear cell renal cell carcinoma developed headache, tinnitus, and severe vision loss (20/200 Snellen equivalent) after 6 cycles of pembrolizumab (400 mg IV every 6 weeks).
- Ocular Findings:
- Anterior chamber and vitreous cells, indicating anterior and posterior segment inflammation.
- Papillitis (optic disc inflammation) and serous retinal detachment bilaterally.
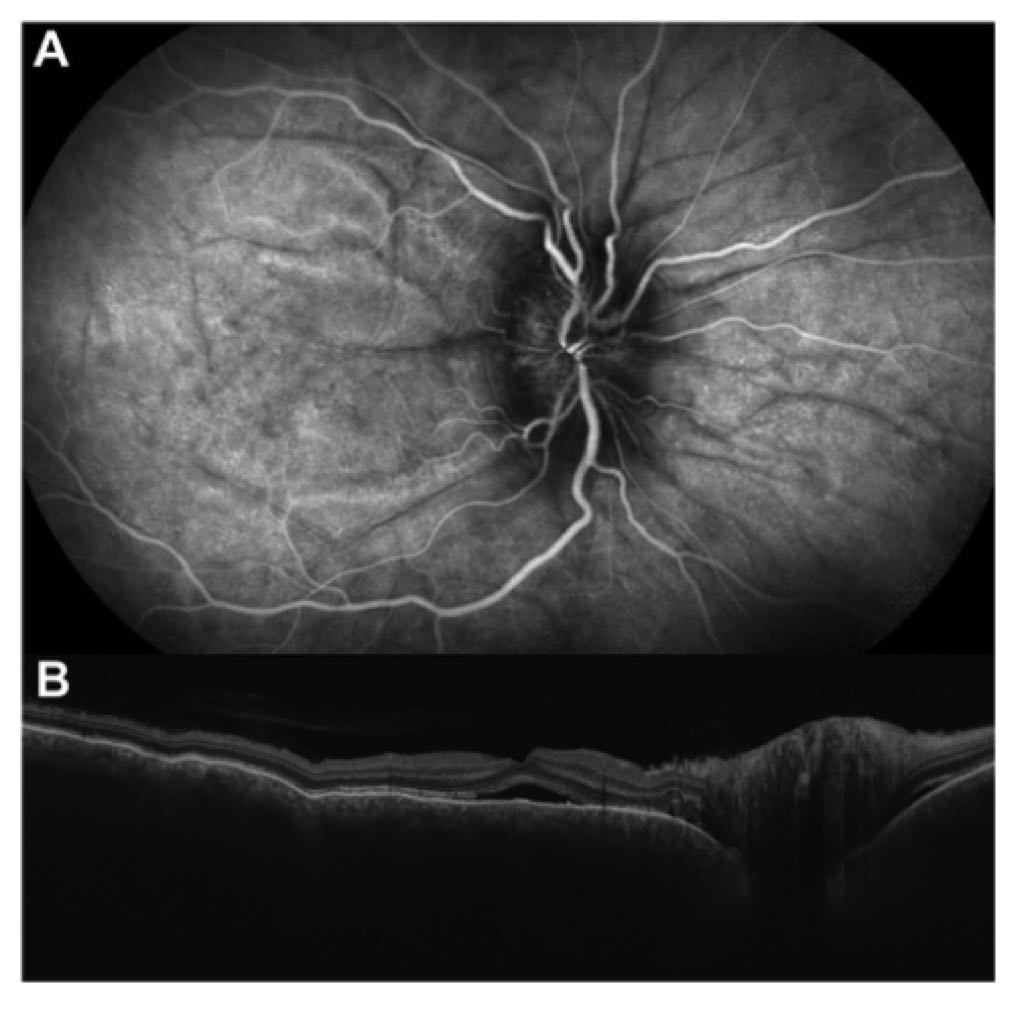
- Choroidal folds, a hallmark feature, visualized as dark lines on midphase indocyanine green angiography (ICGA) against background choroidal fluorescence.
- Subretinal fluid and diffuse choroidal thickening with chorioretinal folds observed on optical coherence tomography (OCT).
- Etiology: Checkpoint inhibitor (pembrolizumab)-induced Vogt-Koyanagi-Harada (VKH)-like uveitis, a rare but serious immune-related adverse event.
- Diagnostic Imaging:
- ICGA: Shows dark lines corresponding to choroidal folds, a critical diagnostic clue.
- OCT: Confirms subretinal fluid, choroidal thickening, and chorioretinal folds, essential for assessing posterior segment involvement.
- Clinical Relevance: Checkpoint inhibitors (e.g., pembrolizumab) can mimic VKH syndrome, requiring prompt recognition to manage vision-threatening complications.
- Management Implication: Severe vision loss and inflammatory findings necessitate urgent ophthalmologic evaluation and potential cessation or modification of immunotherapy, alongside anti-inflammatory treatment (e.g., corticosteroids).
Citation
Carreira, P., Vaz, M., & Cabral, D. (2024). Choroidal Folds in Checkpoint Inhibitor-Induced Vogt-Koyanagi-Harada-Like Uveitis. Ophthalmology Retina, e50. https://doi.org/10.1016/j.oret.2024.10.002